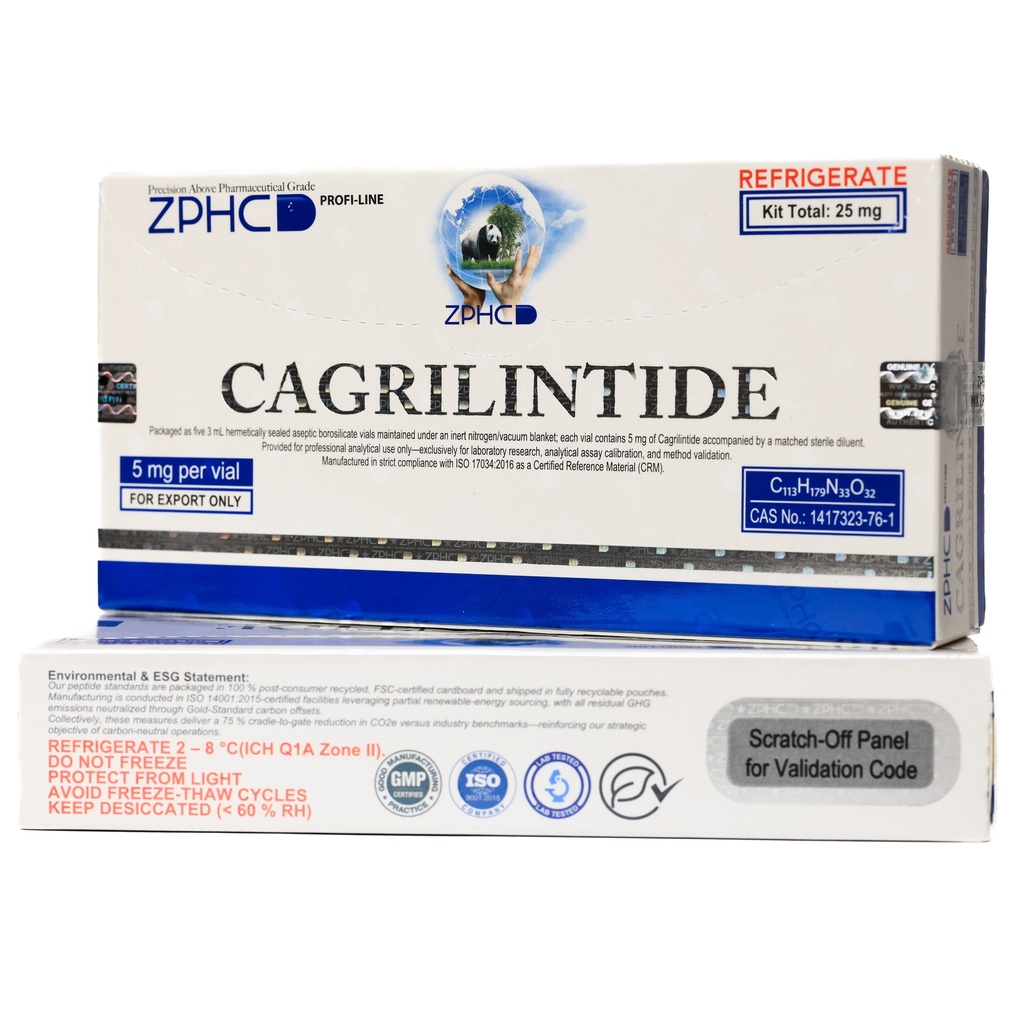
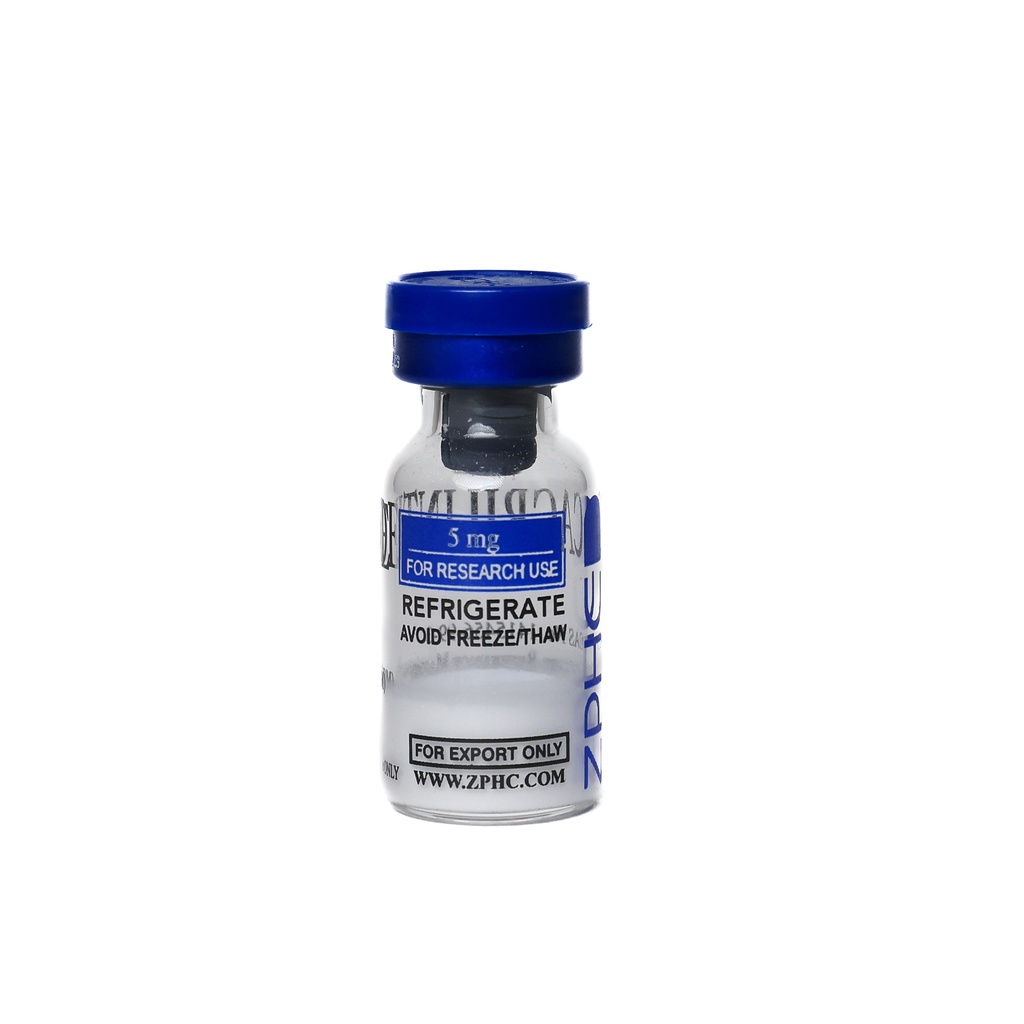
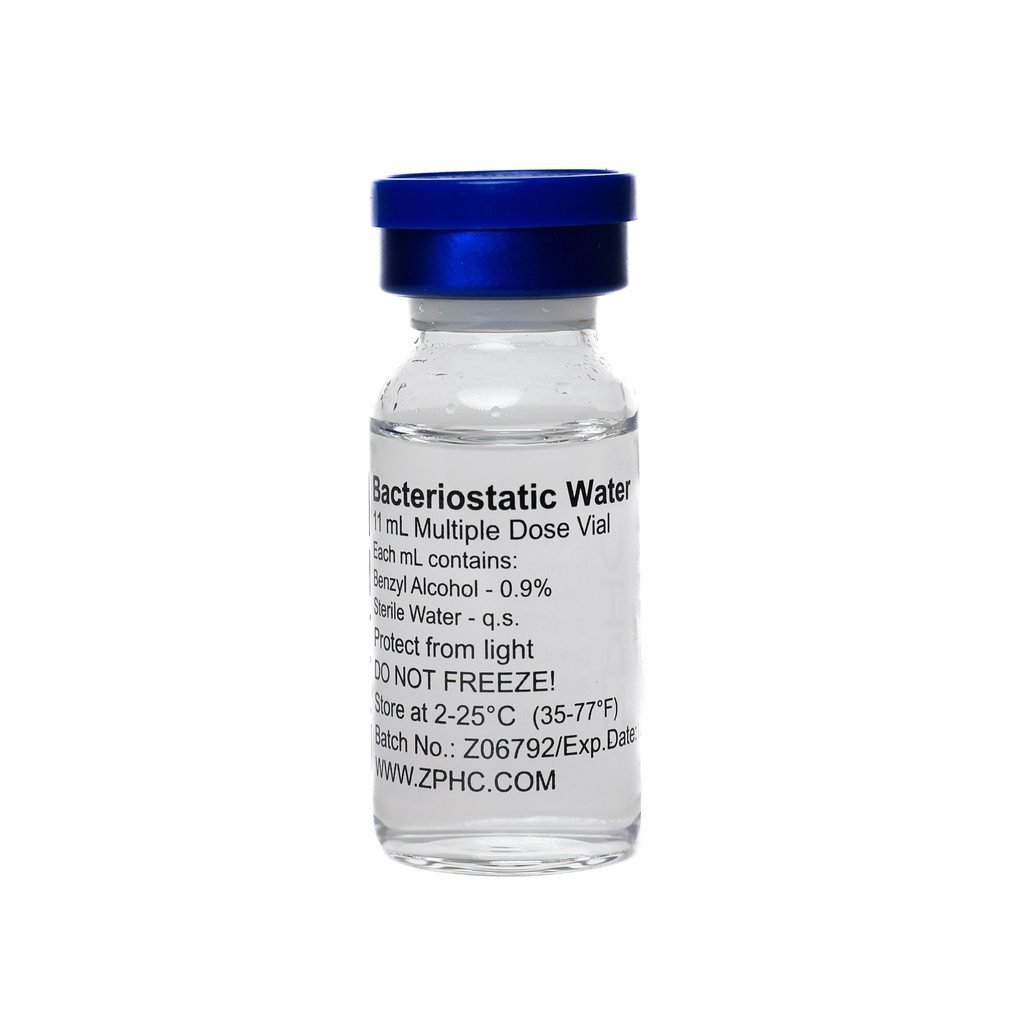
Cagrilintide 25mg -5 MG / VIAL -5 VIALS
Description:
Dosage:
5 MG / VIALQuantity:
5 VIALSWeight:
0.07500 KG
ZPHC PHARMA
Zhengzhou Pharmaceutical (ZPHC) is familiar for its stringent quality control standards as well as laboratory-tested preparations, guaranteeing safe and effective medicine and solutions.
💠 Cagrilintide (AM833) – Science-Based Overview
🔬 What is Cagrilintide?
- Cagrilintide is a synthetic, long‑acting analog of amylin, engineered to activate both amylin and calcitonin receptors for prolonged satiety and metabolic regulation (pep-pedia.org).
- It is lipidated to resist enzymatic degradation and maintain effective plasma levels with once-weekly dosing (Peptide Sciences).
⚙️ Mechanism of Action
- Acts centrally in the hypothalamus to reduce appetite, slow gastric emptying, and suppress glucagon secretion (Peptides.org).
- The dual agonist profile (AMYR and CTR) enhances fullness signals and metabolic stabilization over several days per injection (pep-pedia.org).
✅ Primary Research Applications
| Objective | Outcome & Notes |
|---|---|
| Weight management | Dose-dependent weight loss: ~6–10.8% over ~26 weeks (Peptides.org) |
| Combination therapy (CagriSema) | ≈‑17% weight reduction with 2.4 mg/week + semaglutide versus ≈‑9.8% with semaglutide alone (Peptides.org, Peptides.org, Peptides.org) |
| Glycemic control in T2D | HbA1c reduction up to −2.2%, fasting glucose down ~3.3 mmol/L (Peptides.org, Peptides.org, Peptides.org) |
| Metabolic & cardiovascular effects | Ongoing phase 3 (REDEFINE, REIMAGINE); exploring long-term impact on metabolic risk (Peptides.org, clinicaltrials.eu, pep-pedia.org) |
💉 Recommended Research Dosage Protocols
- Administration: subcutaneous injection once weekly (pep-pedia.org).
-
Titration schedule (commonly used in trials):
- Weeks 1–4: 0.25 mg/week
- Weeks 5–8: 0.5 mg/week
- Weeks 9–12: 1.0 mg/week
- Weeks 13–16: 1.7 mg/week
- Weeks 17+: maintenance at 2.4 mg/week (max) (Peptides.org).
- Monotherapy range: 0.3–4.5 mg/week with higher doses producing greater weight loss (~10.8%) (Peptides.org).
⚠️ Precautions
- Strictly for research only: Not approved or authorized for general human use.
- Avoid abrupt dose escalation; follow gradual titration to minimize gastrointestinal effects (Peptides.org, recessrx.com).
- Not suitable for pregnant individuals, those with gastroparesis, or personal/family history of medullary thyroid carcinoma or MEN2 because of theoretical receptor link risks (recessrx.com, Peptides.org).
❗ Side Effects & Tolerance
- GI-related (dose-dependent): nausea (~47%), vomiting, early satiety, constipation – highest at 4.5 mg/week (Peptides.org).
- Injection-site reactions (redness, discomfort) in ~43% at highest doses (Peptides.org, Peptides.org).
- Other: fatigue (~20%), headache (~7%), loose stool (~7%), dyspepsia (~4%), rare allergic reactions (~10%) (Peptides.org).
- Serious adverse events occurred in 2–7% of participants; discontinuation rate ~10% overall in trials with high-dose arms (Peptides.org, Peptides.org).
🧾 Technical Summary
| Attribute | Details |
|---|---|
| Compound Name | Cagrilintide (AM833, GLXC‑26801) |
| Structure | Lipidated, long‑acting amylin analog targeting AMY and CTR receptors |
| Form | Lyophilized powder; requires reconstitution with sterile bacteriostatic water |
| Frequency | Once-weekly subcutaneous injection |
| Dose escalation regimen | 0.25 → 0.5 → 1.0 → 1.7 → 2.4 mg/week over ~16 weeks |
| Effectiveness | Monotherapy: ~6–10.8% weight loss; Combined: up to ~17% |
| Glycemic benefits | HbA1c reduction ~2.2%; fasting glucose drop ~3.3 mmol/L |
| Storage | Typically frozen (e.g. −20 °C) until use; refrigerate vials after reconstitution |
| Research-only status | Not approved by regulatory authorities for general clinical use |
📌 Final Note
When used in injectable or systemic research settings, Cagrilintide must be administered under the supervision of a qualified medical professional.
| Brands | ZPHC PHARMA |